1. Process Systems Engineering (PSE)
Our research in Process Systems Engineering (PSE) focuses on the development of advanced modeling frameworks, including mechanistic models based on first principles, data-driven models using statistical and AI methods, and integrated hybrid models combining these approaches. We adopt a “multiscale perspective” conscious of the connections from molecules/cells to manufacturing processes and to broader social systems. Our work uses process simulations and assessment to develop decision-support tools with applications spanning health care, clean energy, and sustainable industrial practices.
Process Systems Engineering
Modeling 
Simulation 
Optimization
for Pharma & Sustainability
Pharma PSE 
Sust PSE
2. Pharmaceutical process systems engineering (Pharma PSE)
We are developing methodologies to produce life-saving pharmaceuticals through advanced manufacturing processes. We model production processes across different drug modalities, including small molecules, biopharmaceuticals, and stem cells, with the aim of determining design spaces and optimal operating conditions through detailed simulations. The research was initiated in 2013. Most recently, we began social-scale research by launching “systems med & pharma” as a new theme, which considers manufacturing processes as an element of the social healthcare system.
Please see also the following review papers for more information.
- Gaddem, et al., “Roles of mechanistic, data-driven, and hybrid modeling approaches for pharmaceutical process design and operation” Curr Opin Chem Eng, 44 (2024)
- Badr & Sugiyama, “A PSE perspective for the efficient production of monoclonal antibodies: Integration of process, cell, and product design aspects”, Curr Opin Chem Eng, 27, 121–128 (2020)
Small molecules 
Biopharmaceuticals 
Regenerative medicine 
Systems med&pharma
Research theme and approach
Small molecules
Small molecule drugs contain active pharmaceutical ingredients (APIs) with molecular weights of several hundred. Usually, APIs are synthesized through organic reactions, and the drug products are provided as tablets, capsules, or injections, etc. Currently we are focusing on flow chemistry of the APIs and point-of-care manufacturing of tablets.
Modeling and design space determination for flow syntheses of drug substances
Key publications:
- Kim, et al., “Kinetic study and model-based design space determination for a drug substance flow synthesis using amination reaction via nucleophilic aromatic substitution”, Org Proc Res Dev, 28, 5, 1793–1805 (2024)
- Kim, et al., “Hybrid modeling of an active pharmaceutical ingredient flow synthesis in a ring-opening reaction of an epoxide with a Grignard reagent”, Ind Eng Chem Res, 62, 43, 17824–17834 (2023)
- Kim, et al., “Model-based comparison of batch and flow syntheses of an active pharmaceutical ingredient using heterogeneous hydrogenation”, Comput Chem Eng, 156, 107541 (2022)
Continuous manufacturing & table-top point-of-care manufacturing of solid dosage forms
Key publications:
- Hayashi, et al., “Development of concentration prediction models for personalized tablet manufacturing using near-infrared spectroscopy”, Chem Eng Res Des, 199, 507–514 (2023)
- Matsunami, et al., “Determining key parameters of continuous wet granulation for tablet quality and productivity: A case in ethenzamide”, Int J Pharm, 579, 119160 (2020)
- Matsunami, et al., “Decision support method for the choice between batch and continuous technologies in solid drug product manufacturing”, Ind Eng Chem Res, 57, 9798–9809 (2018)
Biopharmaceuticals
Biopharmaceuticals contain macromolecules with molecular weights ranging from tens to hundreds of thousands as the APIs. The APIs are manufactured through biotechnologies such as genetic recombination and cell culture, and the drug products are usually provided as injectables. Antibody drugs, in particular, are attracting attention as treatments for cancer, autoimmune diseases, and Alzheimer's disease. The market has been expanding rapidly in recent years. Currently we are working on the upstream and downstream processes of therapeutic proteins as well as the sterile filling processes of injectables.
Modeling and simulation of therapeutic antibody manufacturing processes
Key publications:
- Okamura, et al., “Modeling of cell cultivation for monoclonal antibody production processes considering lactate metabolic shifts”, Biotech Prog, e3486 (2024)
- Okamura, et al., “Hybrid modeling of CHO cell cultivation in monoclonal antibody production with an impurity generation module”, Ind Eng Chem Res, 61, 14898–14909 (2022)
- Amasawa, et al., “Cost-benefit analysis of monoclonal antibody cultivation scenarios in terms of life cycle environmental impact and operating cost”, ACS Sust Chem Eng, 9, 14012–14021 (2021)
Manufacturing technology modeling and process data analytics for injectable manufacturing
Key publications:
- Yamada, et al., “A systematic techno-economic approach to decide between continuous and batch operation modes for injectable manufacturing” Int J Pharm, 613, 121353 (2022)
- Zürcher, et al., “Data-driven equipment condition monitoring and reliability assessment for sterile drug product manufacturing: method and application for an operating facility”, Chem Eng Res Des, 188, 301–314 (2022)
- Zeberli, et al., “Data-driven anomaly detection and diagnostics for changeover processes in biopharmaceutical drug product manufacturing” Chem Eng Res Des, 167, 53–62 (2021)
- Shirahata, et al., “Multiobjective decision-support tools for the choice between single-use and multi-use technologies in sterile filling of biopharmaceuticals” Comput Chem Eng, 122, 114–128 (2019)
- Yabuta, et al., “Design-oriented regression models for H2O2 decontamination processes in sterile drug product manufacturing considering rapidity and sterility” Int J Pharm, 548, 466–473 (2018)
- Casola, et al., “Uncertainty-conscious methodology for process performance assessment in biopharmaceutical drug product manufacturing” AIChE J, 64, 1272–1284 (2018)
Regenerative medicine
Regenerative medicine uses human-derived cells, etc., for medical applications. They are attracting attention as a means of enabling therapeutic effects that were not possible with conventional drugs, by culturing iPS cells, mesenchymal stem cells, etc., and differentiating to tissues and organs, or by directly administering the cells. We are investigating unit operations for producing iPS and mesenchymal stem cells.
Design of freezing processes for human induced pluripotent stem cells
Key publications:
- Hayashi, et al., “Computer-aided exploration of multiobjective optimal temperature profiles in slow freezing for human induced pluripotent stem cells”, Cryobiolog, 115, 104885 (2024)
- Scholz, et al., “Computational Fluid Dynamics Model-based Design of Continuous Forced Convection Freezing Processes for Human Induced Pluripotent Stem Cells Considering Supercooling of Extracellular Solutions” Chem Eng Res Des, 208, 674–682 (2023)
- Hayashi, et al., “Slow freezing process design for human induced pluripotent stem cells by modeling intracontainer variation”, Comput Chem Eng, 132, 106597 (2020)
Design of cultivation processes for mesenchymal stem cells
Key publications:
- Hirono, et al., “Image-based hybrid model incorporating initial spatial distribution for mesenchymal stem cell cultivation process design”, AIChE J, 70, e18452 (2024)
- Hirono, et al., “A dynamic and probabilistic design space determination method for mesenchymal stem cell cultivation processes”, Ind Eng Chem Res, 61, 7009–7019 (2022)
Systems med & pharma
We are working on the evaluation and design of social healthcare systems from the perspective of “what is the whole system with manufacturing as an element?”. So far, we have worked on cost-effectiveness analysis, supply chain assessment, and mathematical modeling for infectious diseases. We have initiated “systems med & pharma subdivision” within the Society of Chemical Engineers, Japan to drive our activities.
Key publications:
- Nemoto, et al., “Indicator-based assessment of potential supply risks for pharmaceutical excipients: Method and application”, Int J Pharm, 663, 124498 (2024)
- Okamura, et al., “A systems evaluation model for the development of companion diagnostics and associated molecularly targeted therapies”, J Pharm Innov, 199, 507–514 (2023)
- Kim, et al., “Determination of critical decision points for COVID-19 measures in Japan”, Sci Rep, 11, 16416 (2021)
3. Sustainability process systems engineering (Sust PSE)
Our research employs multiscale process modeling, assessment, and optimization to drive the smart and sustainable development of modern societies. By taking a holistic approach, we tackle the interconnected challenges of increasing demand for energy and materials, climate change, and finite resources. We address these issues from a techno-economic, environmental, and social perspective through an integrated process systems framework.
The research was initiated in 2023, and the current themes are as follows:
- Energy systems and hydrogen society
- Circular economy and waste reduction
- Integrated infrastructure development

Hydrogen society 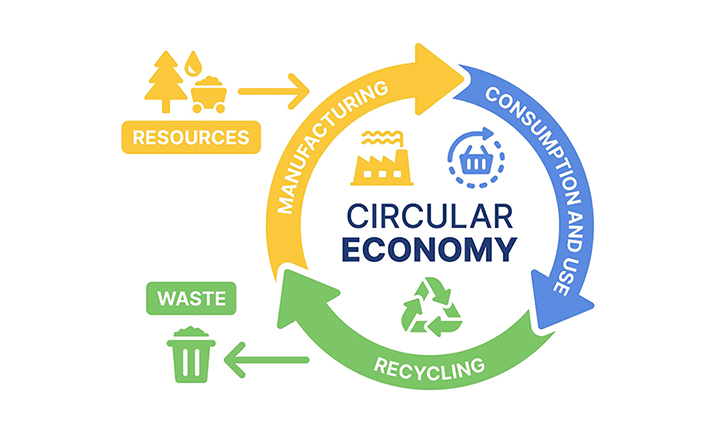
Circular economy 
Infrastructure
Research theme and approach
Energy systems and hydrogen society
To reduce our reliance on fossil fuels, we need alternative energy sources and sustainable chemical processes. Hydrogen is essential in many chemical supply chains and plays a crucial role in energy production and storage.
We are working to unlock the potential of green hydrogen to cut emissions and promote a cleaner future. High production costs remain a significant challenge. Our research focuses on designing integrated hydrogen production processes and exploring breakthroughs in materials and technologies to make these solutions safe, sustainable, and cost-effective. We are also working to ensure a stable hydrogen supply for various use scenarios—whether as a raw material, energy source, or for energy storage. By addressing these challenges, we aim to make green hydrogen a practical tool for a low-carbon world.
Circular economy and waste reduction
A circular economy is key to reducing waste and promoting sustainable production and consumption. By treating waste as a source of energy and materials, we can lessen reliance on fossil fuels and mitigate the environmental impact of persistent waste like plastics. Efficient metal recycling is also vital for scaling green energy technologies, which often depend on scarce metals.
Our research aims to close the loop between production and consumption by developing innovative recycling processes, designing specialized equipment, and creating strategies for technology scale-up and real-world implementation.
Integrated infrastructure development
Integrated infrastructure development is essential for ensuring reliable distribution of energy, water, and resources. Emerging technologies are crucial to tackling challenges like climate change, however, their low readiness levels introduce uncertainty and complicate infrastructure planning. Building infrastructure is a costly, long-term process, and incorporating these technologies requires adaptable planning.
Our research focuses on developing models for large-scale optimization, allowing for comprehensive and prospective assessments across the value chain. By accounting for resource limitations, consumption needs, and technology readiness, we aim to optimize investments, reduce costs, and drive integrated long-term solutions on both regional and global scales.